X Chromosome Inactivation is a fascinating biological process that ensures females, with their two X chromosomes, do not express twice the amount of X-linked genes as males. This essential chromosomal silencing mechanism allows one copy of the X chromosome to be silenced, ensuring genetic balance in female cells. Researchers, like those in Jeannie Lee’s lab, have delved into the intricacies of this complex operation, seeking to unravel its secrets. Their groundbreaking work not only aids our understanding of X chromosome research but also offers hope for innovative treatments for conditions such as Fragile X Syndrome and Rett Syndrome. As discoveries unfold, the potential for gene therapy becomes clearer, shedding light on new avenues for therapeutic advancements in genetic disorders influenced by X-linked mutations.
The intricate process known as X chromosome inactivation plays a vital role in maintaining genetic harmony in female cells by silencing one of the two X chromosomes they possess. This chromosomal silencing is crucial not only for proper gene expression but also in understanding various genetic disorders linked to the X chromosome. In the realm of genetic research, the work being conducted by scientists in the Jeannie Lee lab exemplifies the strides being made towards unraveling these complexities. Their investigations not only contribute to X chromosome research but also hold significant implications for potential Fragile X Syndrome treatments and advancements in Rett Syndrome gene therapy. As we explore the mechanisms behind this process, we may unlock transformative therapeutic options for conditions caused by mutations on the X chromosome.
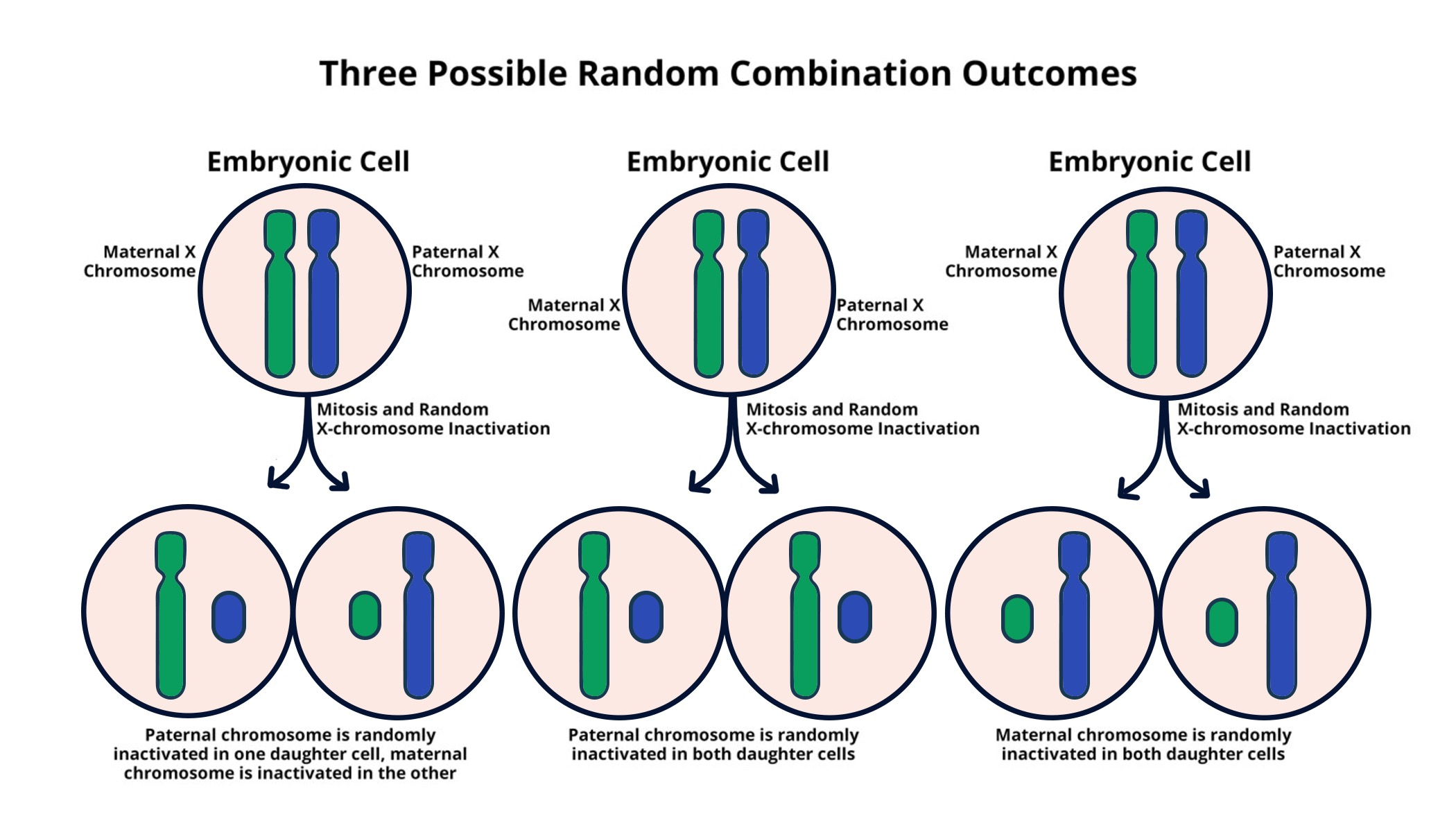
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process that balances gene dosage between males and females by silencing one of the two X chromosomes in females. This process is essential for normal development and function, as it prevents an overexpression of the genes located on the X chromosome. The mechanism behind XCI has long been a topic of research, with scientists like Jeannie T. Lee leading the exploration of chromosome dynamics. The recent findings from her lab have illuminated how chromosomal silencing operates, revealing the roles of specific RNA molecules and surrounding structures that facilitate this complex process.
The research led by Lee’s team has brought to light not only the biological intricacies involved in X chromosome inactivation but also potential therapeutic applications. By understanding how Xist RNA modifies the gel-like substance surrounding the X chromosome, scientists can now consider strategies to reactivate silenced genes that harbor mutations linked to conditions like Fragile X syndrome. This groundbreaking knowledge opens the door for innovative treatments that may shift the management of these genetic disorders.
Innovative Treatments for Fragile X Syndrome and Rett Syndrome
The work being conducted at Jeannie Lee’s lab extends beyond fundamental research; it offers hope for effective treatments for conditions such as Fragile X syndrome and Rett syndrome. Fragile X syndrome is characterized by developmental delays and cognitive impairments, caused by mutations on the X chromosome. Through advancements in understanding XCI, Lee’s team is developing techniques aimed at reactivating the functional genes that are otherwise silenced by their location on the inactive X chromosome. These techniques hold the potential to restore normal gene function, offering a viable pathway for treatment.
Similarly, Rett syndrome, another X-linked neurodevelopmental disorder, shares a genetic basis with Fragile X syndrome. Lee’s lab has identified means to unblock the genes responsible for these conditions, leveraging insights into how chromosomal silencing works. The potential to transition from laboratory findings to clinical trials underscores the promise of this research, aiming to create safe and effective therapies for individuals affected by these debilitating conditions.
The Role of Jaell-O-like Substance in Chromosomal Functions
The gelatinous substance compared to Jell-O plays a pivotal role in the mechanics of X chromosome inactivation. This unique material forms a semi-viscous environment that facilitates the association of silencing factors with the X chromosome, allowing processes that modulate its activity. According to Lee’s findings, the properties of this gel-like substance are altered in such a way that they allow specific molecules, like Xist RNA, to penetrate and modify the chromosome. This nuanced understanding of how the chromosomal silencing mechanism operates is critical for comprehending various genetic disorders.
Moreover, the manipulation of this Jell-O-like substance could present novel therapeutic strategies for gene therapies aimed at disorders linked to the X chromosome. By creating methods to adjust the biophysical properties of this substance, researchers might enhance or inhibit gene activity in targeted ways, which could ultimately lead to new treatments for diseases such as Fragile X and Rett syndrome. This represents a significant leap in the field of genetics, enabling more precise and effective interventions.
Exploring the Mechanisms of Chromosomal Gene Reactivation
The systems governing the reactivation of genes on the inactive X chromosome are complex yet fascinating. Jeannie Lee’s lab has focused on the interactions between Xist and other molecules that contribute to chromosomal silencing and reactivation. Understanding these molecular interactions not only unravels the intricacies of X chromosome biology but also points towards significant therapeutic avenues. The potential to selectively restore function to mutated genes will be transformative in the treatment of genetic disorders.
As researchers continue to decipher the mechanisms of X chromosome reactivation, promising discoveries about gene function and cellular behavior emerge. This research offers insight into why some genes can resume functionality while others remain silent, revealing the delicate balance of gene expression. Unlocking these mysteries will increase the effectiveness of clinical applications targeting Fragile X syndrome and Rett syndrome, bridging the gap between fundamental science and patient care.
X Chromosome Research and Its Implications in Gene Therapy
X chromosome research has significant implications for the future of gene therapy, particularly for diseases that disproportionately affect individuals with XX chromosomal configurations. The breakthroughs associated with X chromosome inactivation provide a foundation for developing new gene therapies aimed specifically at harnessing the power of inactivation and reactivation strategies. By focusing on the pathways identified by researchers like Jeannie Lee, medical science could forge ahead with therapies that allow for the dynamic adjustment of gene expression.
Both Fragile X syndrome and Rett syndrome exemplify the types of conditions that can benefit from advancements in our understanding of the X chromosome. By leveraging innovative gene therapies that target the reactivation of silenced genes, scientists can develop treatments that not only improve symptoms but also address the underlying genetic causes of these disorders. The future of X chromosome research looks promising as it continues to evolve towards practical applications that significantly enhance patient outcomes.
Safety and Efficacy in X Chromosome Reactivation Studies
As research progresses, the factors of safety and efficacy take center stage in the context of developing therapies that reactivate X-linked genes. Jeannie Lee’s lab is not only focused on how to reactivate these genes but also on ensuring that such interventions do not produce adverse effects on healthy genes present on the same chromosome. This meticulous approach to research is critical, as any therapeutic strategies need to balance effectiveness with the potential for unintended consequences that could arise from altering gene activity.
Conducting rigorous safety studies over the upcoming years is essential before transitioning any findings into clinical trials. This phase will involve detailed assessments to evaluate how reactivation affects overall gene function and whether it imposes any risk to patients, including those inheriting other X-linked mutations. The pathway from laboratory discovery to applied clinical practice requires a comprehensive understanding of these dynamics to ensure successful translation into treatments for conditions like Fragile X and Rett syndrome.
Long-Term Goals for X Chromosome Therapeutics
The long-term goals for therapeutic interventions targeting the X chromosome focus on widely applicable strategies that can deliver significant benefits to patients suffering from genetic disorders. Researchers at Jeannie Lee’s lab are optimistic about the potential to modify existing therapies and develop novel approaches that restore the activity of inactivated genes. Each advancement in understanding X chromosome inactivation brings them closer to achieving these long-term goals, with the hope of establishing both effective and durable treatments.
To bring these therapies to fruition, the collaboration among researchers, clinical practitioners, and regulatory bodies will be crucial. Establishing best practices and guidelines for implementing the insights gained from X chromosome research will foster a seamless transition from lab to clinic. As more breakthroughs emerge from ongoing investigations into chromosomal silencing mechanisms, the potential to effectively manage conditions like Fragile X and Rett syndromes continues to grow.
Future Insights into X Chromosome Gene Functions
As the investigation into X chromosome gene functions advances, many exciting possibilities for future insights emerge. With each new discovery concerning XIST and the dynamics of chromosomal silencing, researchers are better equipped to explore how these mechanisms can be manipulated for therapeutic benefit. A deeper understanding of the functional roles that genes play on the X chromosome will allow for the identification of new targets for treatment, improving patient outcomes while minimizing side effects.
Additionally, future studies may also reflect on the evolutionary aspects of X chromosome inactivation and its implications for sex-specific disorders. This understanding might offer broader insights into the genetic basis of diseases that affect males and females differently, leading to tailored treatment options that consider the unique genetic framework of each individual. As researchers continue their efforts, the potential for transformative discoveries in X chromosome biology remains vast.
The Importance of Funding in X Chromosome Research
Sustaining long-term funding for X chromosome research is critical for unlocking future therapeutic options for genetic disorders. As highlighted in the work of Jeannie Lee and her colleagues, initial funding by the National Institutes of Health has allowed for over two decades of investigation into crucial questions surrounding X chromosome inactivation. Continued investment in this field will ensure that crucial questions continue to get addressed, paving the way for clinical applications that hold tremendous promise for patients suffering from conditions like Fragile X syndrome and Rett syndrome.
In addition to governmental funding, collaborations with private sector stakeholders may also play a vital role in directing the future of X research. By fostering partnerships that capitalize on the intersection of scientific exploration and therapeutic development, the community can amplify efforts to translate basic science into tangible health benefits. With adequate resources, the potential for developing innovative treatments that harness the mechanisms of X chromosome inactivation can bring hope to affected families.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in X chromosome research?
X chromosome inactivation is a biological process in females where one of the two X chromosomes is silenced to balance gene expression with males who have only one X. This chromosomal silencing mechanism is crucial in X chromosome research because it helps to understand genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does the Jeannie Lee lab contribute to understanding X chromosome inactivation?
The Jeannie Lee lab at Harvard Medical School has made significant strides in uncovering the mechanisms of X chromosome inactivation. Their research focuses on how the RNA molecule Xist interacts with chromosomal silencing mechanisms to enable this process, which is essential for developing potential treatments for X-linked genetic disorders.
What is the role of Xist in X chromosome inactivation?
Xist is a crucial RNA molecule produced by one of the genes on the X chromosome that initiates X chromosome inactivation. It alters the physical properties of chromosomal surroundings, effectively silencing the X chromosome to ensure balanced gene expression between the sexes.
Can reactivating inactivated X chromosomes provide treatments for Fragile X Syndrome and Rett Syndrome?
Yes, reactivating inactivated X chromosomes may potentially cure genetic disorders such as Fragile X Syndrome and Rett Syndrome. By using techniques developed by the Jeannie Lee lab, researchers aim to unlock the healthy gene trapped within the inactive X chromosome, thereby restoring its function and offering therapeutic options.
What challenges does X chromosome inactivation pose for treating X-linked genetic disorders?
X chromosome inactivation poses challenges for treating X-linked genetic disorders because mutations are typically present on only one of the two X chromosomes, which can hinder access to the healthy gene on the inactive chromosome. Understanding and overcoming this silencing mechanism is crucial in developing effective therapies.
What therapeutic approaches are being explored for X-linked disorders based on recent research?
Recent research by the Jeannie Lee lab explores several methods to reactivate X-linked genes for treating conditions like Fragile X Syndrome and Rett Syndrome. By modifying the chromosomal environment and unlocking inactivated genes, these approaches aim to create effective therapies and enter clinical trials.
How might the findings on X chromosome inactivation affect males with X-linked disorders?
While males do not undergo X chromosome inactivation, they may still benefit from findings related to this process, especially for disorders like Fragile X Syndrome. The research suggests that similar mechanisms could be employed to silence specific genes with mutations, potentially leading to new treatments for affected males.
Why is the study of chromosomal silencing mechanisms significant for genetic research?
Studying chromosomal silencing mechanisms, such as X chromosome inactivation, is significant for genetic research because it opens avenues for therapeutic interventions in genetic disorders. Understanding these mechanisms sheds light on how genes are regulated and how we can manipulate them to address deficiencies caused by mutations.
| Key Concept | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes while males have one. To avoid gene dosage imbalance, one X chromosome in females is inactivated. |
| Role of Xist RNA | Xist RNA is crucial for initiating the inactivation process on the X chromosome, altering the surrounding biophysical properties. |
| Mechanics of the Process | The gelatinous substance surrounding chromosomes (similar to Jell-O) plays a significant role in the accessibility and inactivation of X-linked genes. |
| Implications for Genetic Disorders | Reactivating inactivated X chromosomes may provide therapeutic avenues for disorders like Fragile X Syndrome and Rett Syndrome. |
| Ongoing Research | Continued research aims to optimize treatments and prepare for clinical trials, focusing on safely reactivating X-linked gene functions. |
Summary
X Chromosome Inactivation is a crucial biological process that allows females to manage gene dosage by silencing one of their two X chromosomes. This fascinating mechanism not only helps maintain cellular equilibrium but also opens up new avenues for treating genetic disorders linked to mutations on the X chromosome. Breakthroughs by researchers like Jeannie T. Lee highlight the potential for future therapeutic interventions that could reactivate dormant genes and offer hope to those affected by conditions such as Fragile X and Rett Syndromes.